
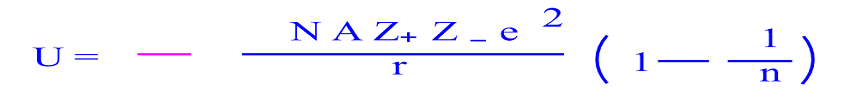
The strength of the bond between the ions of opposite charge in an ionic compound. The energy value can be estimated using the Born-Haber cycle, or it can be calculated theoretically with an electrostatic examination of the crystal structure. Lattice Energies and the Strength of the Ionic Bond.

Lattice energy cannot be determined experimentally due to the difficulty in isolating gaseous ions. An equation is developed for calculating lattice energies, using the most readily available experimental data the results are at least as reliable as those. We will sum the energies of each step and that will be our total. The measured value is compared against the theoretical value obtained by physics calculation. We will use Hesss Law to rearrange the steps so that we get the net equation we are after. NaCl Crystalline LatticeSodium ions (Na +) and chloride(Cl -) ions, depicted in purple and green respectively, alternate in the crystal lattice of solid NaCl. So, the lattice energy for ionic sodium chloride is -786KJ/mol. Molecular simulation method has also been carried out to. For the IB it is defined as the energy released when 1 mole. The simple equation describing the best plot wasUperovskite oxide 1,0579U(YF) - 835,06 kJ/mol. The lattice energy of an ionic compound is the energy change when one mole of ionicsolid is separated into its gaseous ions. The negative sign of the energy is indicative of an exothermic reaction.Īlternatively, lattice energy can be thought of as the energy required to separate a mole of an ionic solid into the gaseous form of its ions (that is, the reverse of the reaction shown above). Lattice enthalpy may be seen defined in two different ways, depending on the reference literature. N a + ( g ) + C l − ( g ) → N a C l ( s ) Δ H = − 787.3 k J / m o l Na^+ (g) + Cl^- (g) \rightarrow NaCl (s) \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \Delta H=-787.3 kJ/mol N a + ( g ) + C l − ( g ) → N a Cl ( s ) Δ H = − 787.3 k J / m o l Lattice Energy or Lattice Enthalpy H L rm Delta HL HL, is the enthalpy associated with the formation of gaseous ions from the solid ionic substance.


 0 kommentar(er)
0 kommentar(er)
